Problem
of the Week
 |

©2000 Australian
Broadcasting Corporation
|
A
Unique Insect
The bombardier beetle is a
small insect whose habitat is under rocks that are found in the moist
flood plains of rivers, lakes, and temporary post-rainstorm ponds of
southern Canada, the United States, and Australia. This unique insect
defends itself by firing a boiling hot spray from the rear of its
abdomen when assaulted. This spray allows the beetle to unfurl its
wings from under wing covers and fly away. The spray is formed at the
moment of firing by mixing chemicals from two glands in the beetle's
abdomen. The spray changes instantly into a gas and is directed away
from the beetle. The gas irritates the eyes of the enemy and forms a
smoke screen that helps the beetle to escape while the enemy is
confused. |
| Two chemicals, hydroquinone and hydrogen peroxide,
collect in a reservoir that has a valve controlled opening into a
thick-walled reaction chamber. The reaction chamber is lined with cells
that secret peroxidases and catalases, enzymes that rapidly break down
the hydrogen peroxide and hydroquinone. When the contents of the
reservoir are forced into the reaction chamber through a
muscle-controlled valve, a reaction quickly occurs. Hydroquinone is
changed into p-quinone and the hydrogen peroxide into water and oxygen.
There is enough heat generated from the reaction to bring the mixture
to the boiling point and vaporize some of it creating pressure in the
reaction chamber. The products of the reaction are expelled explosively
through openings at the tip of the beetle's abdomen. |
| |
 |
 |
 |
Balance the equation for the reaction that occurs in
the reaction chamber of the bombardier beetle.
C6H4(OH)2 (l) + H2O2
(l) → C6H4(OH)2 (l) + H2O
(l) + O2 (g)
Hydroquinone + Hydrogen peroxide → p-quinone + water + oxygen
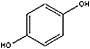 + +  → → 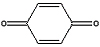 + + 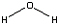 + +  |
| |
 |
How many grams of water can be formed if 1.00 grams of
hydrogen peroxide reacts with 2.00 grams of hydroquinone in the
reaction chamber? |
| |
 |
If 1.00 grams of hydrogen peroxide and 2.00 grams of
hydroquinone are added to the reaction chamber, which is the limiting
reactant? What is the excess reactant and by how much is in excess? |
 |
Useful Web Sites:
No
place to hide
Chemical
Secretions of the Suborder Adephaga (Coleoptera) |
|

